Dr Eric Hewitt
- Position
- Associate Professor
- Areas of expertise
- cell biology; immunology; amyloid; lysosomes; nanoinjection
- Phone
- +44(0)113 343 3030
- Location
- 7.19c Miall
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
- Website
- ORCID
Introduction
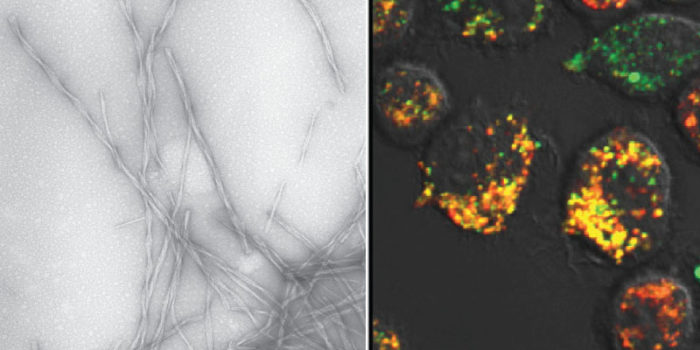
The formation of insoluble amyloid fibrils is associated with a spectrum of devastating human disorders, including Alzheimer’s, Parkinson’s, type 2 diabetes and dialysis related amyloidosis (DRA). Our goal is to determine how the structure and physical properties of amyloid fibrils and their assembly intermediates determines their toxicity and how they activate immune response. This work involves a multidisciplinary approach in which information obtained by structural biology techniques is integrated with cell biological and immunological analyses. Currently, we are studying the oligomeric assembly intermediates and fibrils formed by the amyloidogenic sequences, α-synuclein (Parkinson’s), amyloid-β (Alzheimer’s) and β2-microglobulin (DRA). We are examining the uptake of amyloid aggregates by cells, the interactions between amyloid and cellular components, the induction of inflammatory responses, and the effects of amyloid on cellular physiology and cell viability. These cell based experiments use an array of techniques, including plate-based assays for cell viability and metabolism, live cell confocal microscopy, flow cytometry, ELISA, subcellular fractionation and proteomics. In addition, we are exploring a novel single molecule platform for the delivery of amyloid aggregates into the cytoplasm of cells. Our work is highly collaborative and we work closely with Professors Sheena Radford and Andy Wilson from the Astbury Centre, as well as Dr Paolo Actis from the School Electrical and Electronic Engineering.
Current major projects
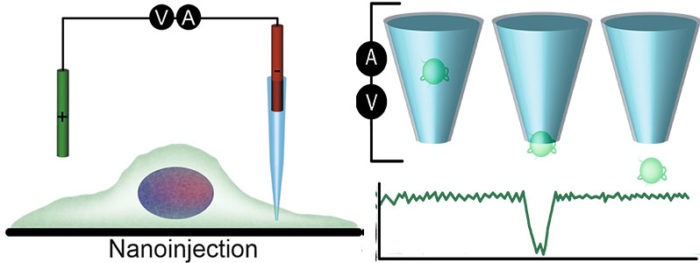
- Nanoinjection: a quantitative delivery platform to study amyloid toxicity inside cells
- Using proteomics to probe cellular interactions with amyloid fibrils
- Analysing the immune response to amyloid fibrils
Detailed research programme
Nanoinjection: a quantitative delivery platform to study amyloid toxicity inside cells
We are using a nanoinjection platform for the quantitative and targeted delivery of amyloid fibrils and oligomers into cells for functional analysis. The platform was developed by Paolo Actis and uses quartz needles with ≤50nm diameter pores to inject macromolecules into cells. Due to the small size of the pore individual macromolecules can be detected when they are delivered into cells, thus cellular delivery can be quantified. Moreover, integration with a surface ion conductance microscope, a confocal microscope and nanomanipulator enables highly precise delivery to specific sites in the cells.
Using proteomics to probe cellular interactions with amyloid fibrils
Characterising the cellular interactions of amyloid fibrils is crucial if we are to understand how amyloid fibrils cause cellular dysfunction and death. This information may also provide insights into how cells can protect themselves from the harmful effects of amyloid fibrils. We are using pulldown proteomics to identify interaction partners of α-synuclein and β2-microglobulim amyloid fibrils. Moreover, we are exploring novel chemical cross-linking methods with Andy Wilson’s lab, with the goal of identifying the interaction partners of fibrils taken up into cells.
Analysing the immune response to amyloid fibrils
We are studying the responses of microglia and macrophages to amyloid fibrils. Our principal focus is amyloid-β fibrils and we are exploring how the molecular structure of these fibrils impacts on the immune response. More specifically, we are analysing the uptake and the lysosomal degradation of these fibrils, as well as examining the ability of these fibrils to promote proinflammatory cytokine production and the presentation of antigens.