Dr George Heath
- Position
- University Academic Fellow
- Areas of expertise
- High-Speed Atomic Force Microscopy; Membrane Proteins; Lipid Membranes; Biophysics; Self-assembly
- Location
- 8.52 EC Stoner
- Faculty
- Engineering and Physical Sciences and Biological Sciences
- School
- Physics & Astronomy and Biomedical Sciences
Introduction
The rapid expansion in the structural understanding of proteins through recent progress in electron microscopy and X-ray crystallography is leading to an ever-greater need for techniques which give high temporal resolution dynamics. High-Speed Atomic Force Microscopy (HS-AFM) provides unprecedented real-space and real-time visualisation of biological molecules (<1 nm lateral, ~0.1 nm vertical and >100 ms temporal resolution). My research interests are focused on developing techniques to study the structure and dynamics of biomolecules at previously inaccessible time and spatial resolutions using atomic force microscopy. In doing this we aim use physics and physical tools to better understand biological processes related to health and disease.
Current major projects
- Developing high-speed atomic force microscopy methods for microseconds and sub-nm resolution
- Conformational dynamics of TRPC ion channels in response to small molecules
- Force response and structural dynamics of mechanosensitive ion channels
Detailed research programme
High-speed-AFM method development
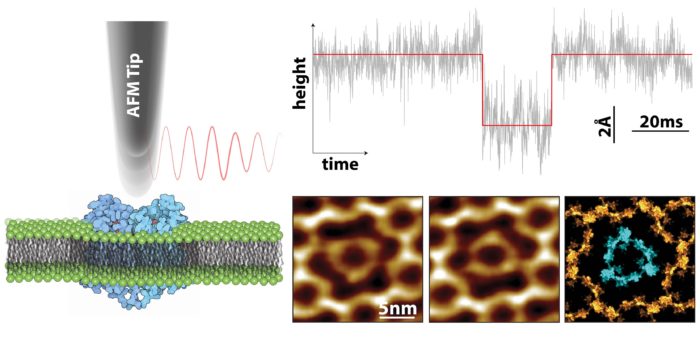
We have recently developed HS-AFM to increase the already pioneering 100ms time resolution to 10μs. This 10,000-fold leap in acquisition rate is achieved by no longer scanning the surface with a tip, but by holding it at a single point of interest and studying the dynamics of molecules underneath. This offers the capability to directly study how protein dynamics can be modulated by various small molecules and stimuli to inform the pursuit of therapeutics.
TRPC ion channel structural dynamics
Transient receptor potential canonical (TRPC) 1/4/5 channels are found in many cell types including the nervous and cardiovascular systems, where they form nonselective cationic channels with high calcium permeability. Structural and mechanical knowledge of these channels is rapidly evolving, suggesting TRPC1/4/5 assemblies play crucial roles in human physiology and pathology, and consequently are potential drug targets for renal failure, anxiety, cancer, pain, and cardiac remodeling. Our goal is to study the dynamic structural changes in TRPC1/4/5 assemblies reconstituted into near-native membranes as a function of modulators by high speed AFM. The mechanisms we aim to uncover will not only be applicable to TRPC but also to all other TRP and heteromeric ion channels.
Pushing and imaging mechanosensitive channels
Mechanosensitive channels are found throughout all kingdoms of life acting as sensors for a number of systems including touch, hearing, gravity, osmotic pressure and cardiovascular regulation. Embedded within lipid membranes, these pressure sensitive channels sense and open upon specific mechanical stimuli allowing certain molecules to enter or exit the cells. In humans they are vital for many physiological functions and a number of specific genetic mutations within these channels have been implicated in several diseases. Despite their fundamental importance, the molecular mechanism of how these channels respond to membrane tension is not well understood. We aim to utilize HS-AFM to both apply force and monitor conformational dynamics of single membrane protein channels in real-time at the nanoscale.