Dr Glyn Hemsworth
- Position
- Associate Professor
- Areas of expertise
- Structural Biology; Enzymes; Redox Processes; Glycobiology; Biotechnology
- Phone
- +44(0)113 343 4349
- Location
- 10.107 Astbury
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
- Website
- ORCID
Introduction
Our laboratory uses an interdisciplinary approach to study protein structure and function using X-ray crystallography coupled with biophysical and biochemical methods. Using these approaches, we can gain a full understanding of how proteins perform their functions so that we can ultimately exploit their properties in various biotechnologies. Overall, we aim to provide protein-based reagents that can be used to reduce pollution and conserve resources to provide a more sustainable means for the manufacture of many chemicals and fuels into the future.
Current major projects
- Discovery and characterisation of novel proteins with potential industrial applications
- Exploitation of plastic binding domains for the enzymatic degradation of plastics
Detailed research programme
Structural and Functional Characterisation of Novel Proteins with Potential for Industrial Biotechnology
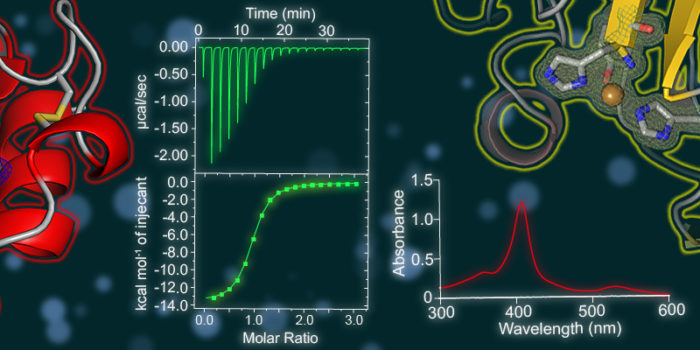
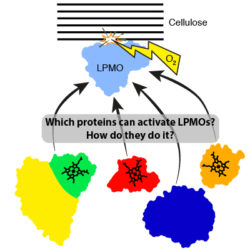 Industrial biotechnology seeks to exploit enzymes and/or organisms to efficiently and sustainably produce many of the chemical precursors and liquid fuels upon which we rely. One of the main aims in this area has been to utilise enzymes to efficiently break down cellulose into glucose which can subsequently be converted into biofuels. New enzymes known as Lytic Polysaccharide Monooxygenases (LPMO) have emerged as key players in cellulose degradation but their action is incompletely understood. We are structurally and biochemically characterising a range of bacterial proteins that may act as activators of LPMOs. Understanding LPMO activation mechanisms can both provide fundamental insight into the reaction mechanism used by these enzymes and suggest the best way to activate these enzymes in industry.
Industrial biotechnology seeks to exploit enzymes and/or organisms to efficiently and sustainably produce many of the chemical precursors and liquid fuels upon which we rely. One of the main aims in this area has been to utilise enzymes to efficiently break down cellulose into glucose which can subsequently be converted into biofuels. New enzymes known as Lytic Polysaccharide Monooxygenases (LPMO) have emerged as key players in cellulose degradation but their action is incompletely understood. We are structurally and biochemically characterising a range of bacterial proteins that may act as activators of LPMOs. Understanding LPMO activation mechanisms can both provide fundamental insight into the reaction mechanism used by these enzymes and suggest the best way to activate these enzymes in industry.
Identification and Exploitation of plastic binding domains
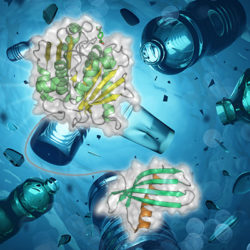 The release of (micro)plastics into our environment represents a major concern globally. Excitingly, microorganisms that are capable of breaking down some plastics have started to be discovered. They have evolved enzymes that can break the bonds in plastic polymers, but it may be possible to improve on these with modern protein engineering approaches. We are currently investigating whether we can engineer small plastic binding domains that can be appended to plastic degrading enzymes to give them better access to their substrate. If the efficiency of enzymatic plastic degradation can be significantly improved then it should be possible to genetically engineer microbes that can be used to bioremediate the effects of plastic pollution.
The release of (micro)plastics into our environment represents a major concern globally. Excitingly, microorganisms that are capable of breaking down some plastics have started to be discovered. They have evolved enzymes that can break the bonds in plastic polymers, but it may be possible to improve on these with modern protein engineering approaches. We are currently investigating whether we can engineer small plastic binding domains that can be appended to plastic degrading enzymes to give them better access to their substrate. If the efficiency of enzymatic plastic degradation can be significantly improved then it should be possible to genetically engineer microbes that can be used to bioremediate the effects of plastic pollution.