Dr Joe Cockburn
- Position
- Lecturer
- Areas of expertise
- Structural biology molecular motors cilia
- Phone
- 01133430758
- Location
- Astbury 8.111
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
Introduction
Broadly speaking, the research of our group aims to understand how the positioning of cellular components is encoded at the molecular level.
The cytoskeleton is a dynamic scaffold inside cells that determines cell shape, allows cells to move, and functions as a set of internal tracks for transport of cellular components (e.g. organelles) by molecular motors. Defects or deregulation of cytoskeletal processes are implicated in various pathogenic states such as viral infections, neurodegeneration, developmental defects and cancer.
We combine structural biology (principally X-ray crystallography and increasingly cryo-EM) with biophysical and cell biology approaches to obtain a unified understanding of the biological processes under study. Our work is enhanced through collaborations with a number of other research groups both in Leeds and elsewhere in the UK and Europe.
Current major projects
- Molecular motors: how cellular cargoes (e.g. organelles, viruses, vesicles) recruit and control (i.e. “drive”) molecular motors (in collaboration with Dr Stuart Warriner, University of Leeds, and Dr Alison Twelvetrees, University of Sheffield)
- The ciliary transition zone: understanding the formation and maintenance of primary cilia (in collaboration with Prof. Colin Johnson, University of Leeds, and Prof. Lotte Bang Pedersen, University of Copenhagen)
- Seminal fluid proteins in Drosophila (in collaboration with Prof Elwyn Isaac, University of Leeds)
Detailed research programme
How cellular cargoes recruit and control molecular motors
The cytoplasm is a highly crowded environment containing tens of thousands of different protein species, mRNA molecules, ribosomes, vesicles and organelles. Cellular function is critically dependent on the correct localisation of these components in space and time.
The movement of cellular cargoes over long distances requires dedicated motor proteins (kinesins and cytoplasmic dynein) that use ATP hydrolysis to power movement along a network of tracks called microtubules. How these motors couple ATP hydrolysis to movement is now fairly well understood, and attention is now turning towards how cellular cargoes recruit molecular motors and regulate their motility. The combined action of all the kinesin and dynein motors inside your body is very powerful – if all the kinesin motors in your cells were working at full tilt all the time, they would use up ~8000 kcal of energy per day! Molecular motors must therefore be carefully regulated by their cargoes to ensure that they only consume energy when needed.
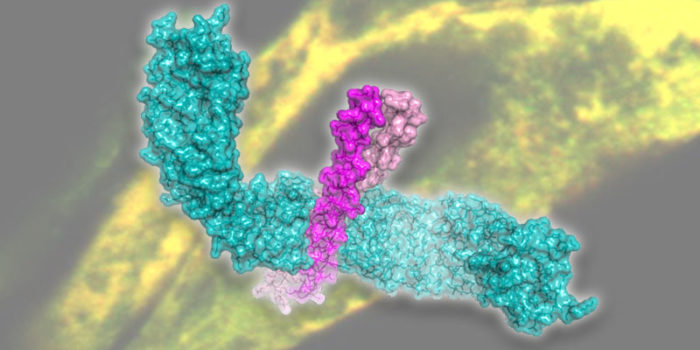
The main focus of our activity at present is on kinesin-1, which mediates the long-range transport of diverse cellular cargoes (proteins, mRNPs, vesicles, organelles and viruses). We solve structures of the cargo-binding regions of kinesin-1 in complex with cellular cargoes (Fig. 1) to gain insights into how kinesin-1 switches itself off when not in use, how cargo molecules bind to kinesin, and how this “switches on” kinesin-1 motility.
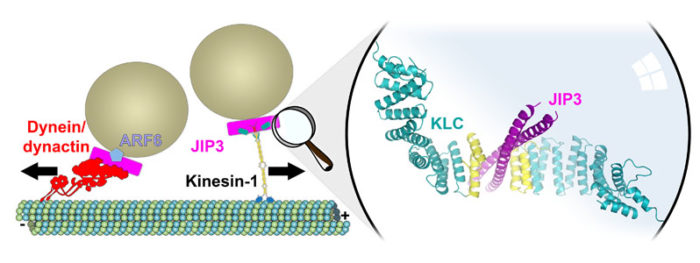
In collaboration with Dr Stuart Warriner in the School of Chemistry, University of Leeds, we are also developing synthetic kinesin-1:cargo pairings, which will provide new tools to study kinesin-1 function inside cells.
The ciliary transition zone: understanding the formation and maintenance of primary cilia
The primary cilium is the “antenna” of eukaryotic cells, allowing us to see, hear, excrete, develop and reproduce. The primary cilium is a specialised compartment of the plasma membrane enriched in receptors and downstream effectors and furnished with its own microtubule transport system. The composition of the ciliary membrane is controlled by a diffusion barrier at the boundary with the plasma membrane (the transition zone) comprising over 20 protein species. Mutations these proteins cause a spectrum of inherited developmental disorders such as Joubert syndrome and Meckel syndrome, characterised by retinal, renal and CNS malformations. There are no treatments or preventative therapies for these conditions.
The structures of transition zone proteins and their organisation at the ciliary base are poorly understood. To address this need, we are developing a program of integrative structural biology research to understand transition zone architecture and function at the molecular level. Our vision is that understanding the molecular architecture of the transition zone will allow us to unravel the complexity in genotype-phenotype associations for these ciliopathy disorders.
To meet this challenge, we have established an interdisciplinary collaboration between the Cockburn and Peckham labs (Astbury), and the Johnson lab at the Faculty of Medicine and Health. This will allow us to obtain high-resolution structures of transition zone proteins and their complexes by X-ray crystallography and/or cryo-electron microscopy, identify how they are organised at the ciliary base inside healthy and patient-derived cells using super-resolution microscopy, and then use these models to formulate and test hypotheses about how mutations disrupt primary cilium function in patient cells and organoids. This will work will inform personalised medicine efforts to treat or prevent ciliopathy conditions.
