Dr Katie Simmons
- Position
- Lecturer in Structural Pharmacology
- Areas of expertise
- medicinal chemistry; chemical biology; ligand design; molecular modelling; molecular biology
- Location
- Garstang 6.44F
- Faculty
- Biological Sciences
- School
- Biomedical Sciences
Introduction
My research group focuses on the identification of bioactive molecules for early-stage lead discovery on a number of challenging targets involved in disease areas such as cardiometabolic disorders, epilepsy and bunyaviruses. We use a range of computational methods to discover novel ligands for these target proteins, coupled with small molecule synthesis and assay development- collaborating with colleagues at the University of Leeds and beyond. Our primary aim is to identify therapeutic leads and to partner with industrial collaborators to further these leads as drug candidates.
Current major projects:
- RGD-mimetics as a new treatment for Type 2 Diabetes
- Identification of Fluorine-containing inhibitors of proline-rich tyrosine kinase and endothelial nitric oxide synthase
- Novel potassium channel inhibitors for the development of treatments for KCNT1-related epilepsy
- Development of modulators of IR-IGF1R hybrids as a potential treatment for Type 2 Diabetes
Detailed research programme
RGD-mimetics as a new treatment for Type 2 Diabetes
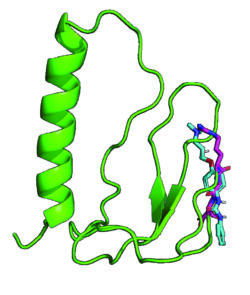
This project builds on the discovery that the RGD-motif of the circulating protein insulin-like growth factor binding protein-1 (IGFBP1) confers insulin sensitisation and protection from cardiovascular disease in mice. Here we investigate lead small-molecule compounds identified through in silico screening to replicate the effects of the RGD-domain of IGFBP1 as integrin-agonists and act as putative insulin-sensitisers. We anticipate that our findings will confirm that targeting integrins using RGD-mimetic agonists is a tractable therapeutic approach in cardiometabolic disease and will generate early stage leads for future drug discovery.
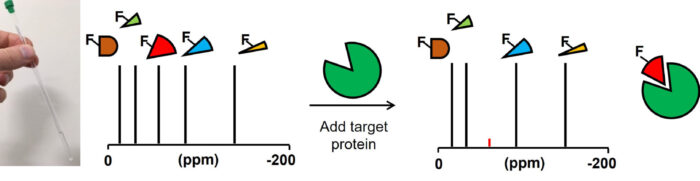
Identification of Fluorine-containing inhibitors of proline-rich tyrosine kinase and endothelial nitric oxide synthase
Fragment-based ligand discovery allows greater sampling of chemical space compared with traditional screening techniques, even with a modestly sized compound library. This project aims to identify fragments which modulate the eNOS-PYK2 interaction, which can be elaborated into small molecules. Because of the weaker binding affinities typically exhibited by fragments, sensitive biophysical techniques such as Nuclear Magnetic Resonance (NMR) are required to detect the binding event. The use of fluorine-containing fragments for NMR screening allows increased throughput as the spectra obtained are simplified and easier to read. This gives us the ability to screen mixtures of samples without spectral overlap, maximising efficiency. This newly discovered interaction offers a novel way to address the cardiovascular complications of type 2 diabetes.
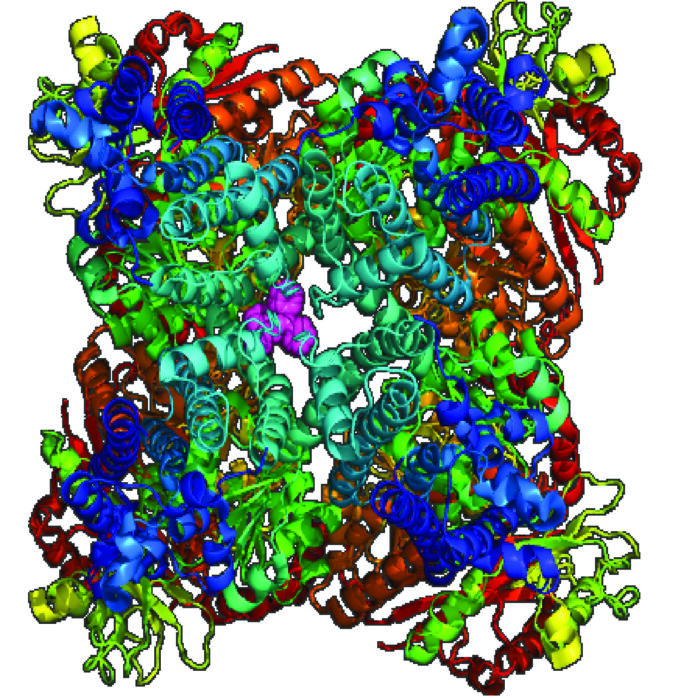
Novel potassium channel inhibitors for the development of treatments for KCNT1-related epilepsy
Inherited variations in the KCNT1 ion channel cause severe childhood epilepsy, with frequent seizures, intellectual and motor disabilities. These rare forms of epilepsy cannot be controlled by any presently available medicine. We have identified novel inhibitors using computer-aided ligand-discovery and are combining medicinal chemistry and molecular biology approaches to develop and test putative new therapeutics to treat epilepsy.
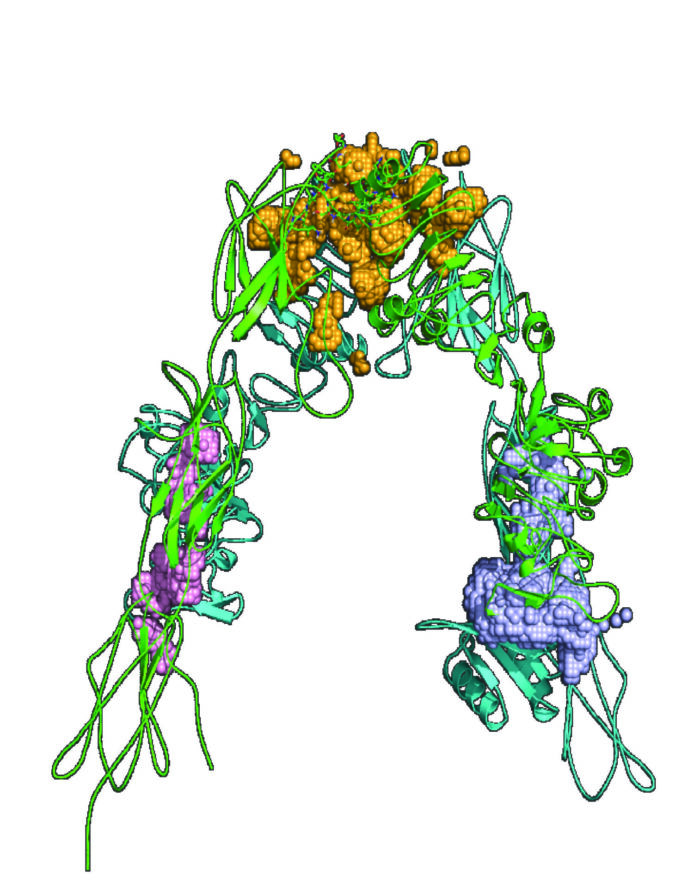
Development of modulators of IR-IGF1R hybrids as a potential treatment for Type 2 Diabetes
The insulin receptor (IR) and insulin like growth factor-1 receptor (IGF1R) are heterodimers consisting of two extracellular α-subunits and two transmembrane β -subunits which can heterodimerize to form hybrids composed of one IR and one IGF1R. Widely distributed in mammalian tissues including the cardiovascular system, the physiological function of hybrids is unclear. This project aims to identify tool compounds that inhibit hybrid formation and begin to understand the role hybrid receptors have in regulating PI3-kinase signalling.