Professor Lorna Dougan
- Position
- Professor of Physics
- Areas of expertise
- biomechanics; single molecule biophysics; hydrogels; extreme biophysics
- L.Dougan@leeds.ac.uk
- Phone
- +44(0) 113 343 8958
- Location
- EC Stoner 8.32
- Faculty
- Engineering and Physical Sciences
- School
- Physics
Introduction
We are a multidisciplinary research group and motivated to understand the physics of life. We are developing tools to explore multiscale scale mechanics, single molecule manipulation techniques and neutron scattering to explore the physics of living systems. These powerful techniques are used to study hierarchical biomechanics, self-assembly and the structure and dynamics of molecules in aqueous solutions, in both simple and complex systems.
Current major projects
- Engineered proteins in the design of robust and tuneable biofunctional materials
- The role of water in extreme biological environments
- Uncovering the stability and dynamics of proteins from extremophilic organisms
- Exploiting hydrogels in novel multicomposite drug delivery systems
Detailed research programme
Engineered proteins in the design of robust and tuneable biofunctional materials
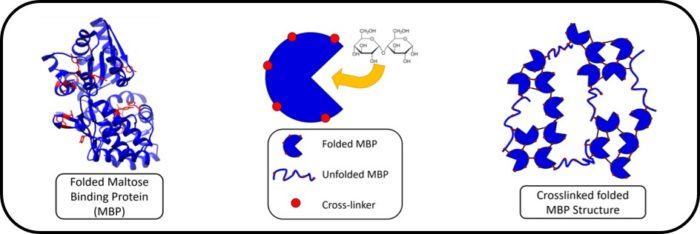
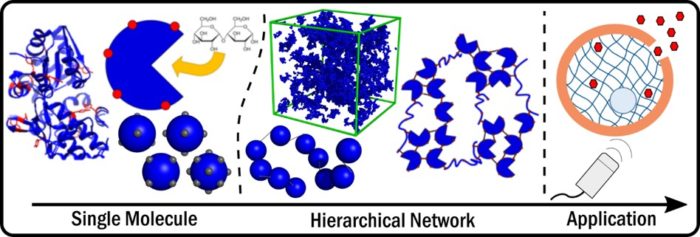
Our group are leading novel research in the area of hierarchical biomechanics: mechanics of biological systems across length scales. We are creating folded polyprotein hydrogels which possess specific biological function capabilities, enabling dynamic changes in mechanical and structural properties in response to biomolecular cues. This is achieved through a cross length scale, physics-based approach which will translate knowledge of the nanoscale biophysics of folded proteins to the mesoscale architecture and function of novel folded polyprotein hydrogels. By understanding the physics of the building block (the folded protein) and its connectivity (the polyprotein network) we are creating a platform for the production of novel, biomaterials. Our work is in collaboration with David Brockwell (Biology) and David Head (Computing).
The role of water in extreme biological environments

There is huge current interest in the role of water in biology under the extreme conditions of temperature, pressure and solvent environment. We combine our extreme biophysics activities at Leeds, with the world leading neutron diffraction facilities of the ISIS neutron and muon facility, as well as computational modelling for the interrogation of total scattering data to yield powerful new insight into water and its role. This allows us to tackle questions, so far unanswered, of extreme adaptation, including the role of water in cryoprotectant molecules which are widely used in basic molecular research through to industrial and biomedical applications and action of trimethylamine N-Oxide (TMAO), a highly effective protein stabilizing agent which induces protein folding, and resists the denaturing effects of pressure. Our work is in collaboration with Alan Soper and Tristan Youngs (ISIS neutron facility, Oxford).
Uncovering the stability and dynamics of proteins from extremophilic organisms
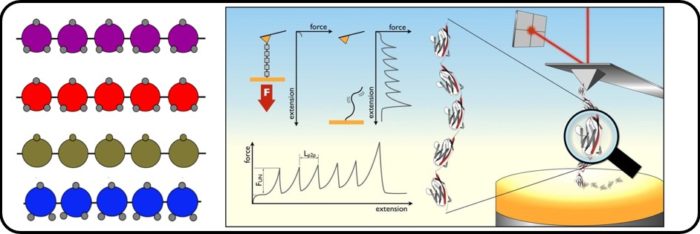
The wonderful ability of organisms to thrive in extreme environments challenges our views of the natural limits of life and offers powerful model systems for understanding the physics of life. Extremophiles are organisms which survive and thrive in extreme environments. The proteins from extremophilic single-celled organisms are structurally stable and functionally active under extreme physical and chemical conditions. We are using single molecule force spectroscopy to mechanically manipulate proteins from extremophilic organisms to gain information about their stability, flexibility and underlying energy landscapes and NMR to examine their interesting dynamics. This is allowing us to unlock the mysteries of these fascinating extremophile organisms. Our work is in collaboration with David Brockwell (Biology)
Exploiting hydrogels in novel multi-composite drug delivery systems
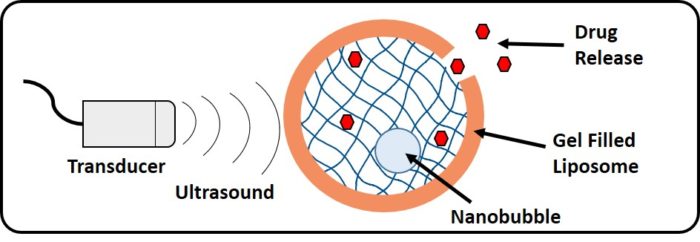
Hydrogels are highly porous, 3D hierarchically structured networks that are swollen with water. We are exploiting hydrogels to biocompatible, responsive materials for triggered drug delivery. Our novel composite system involves liposomes with encapsulated hydrogel embedded with nanobubbles, providing a drug transport mechanism with an ultrasound triggered release system. The lipid bilayer protects the hydrogel from degradation in the bloodstream, as well as reducing the side effects of toxic drugs on healthy cells by targeting to the region of interest. The presence of a drug loaded gel within the liposome core aims to further control the speed that the drug molecules are released. Our work is in collaboration with Steve Evans and Sal Peyman (Physics) and Louise Coletta (Medicine).
