Professor Mike Webb
- Position
- Professor of Biological Chemistry
- Areas of expertise
- Chemical Biology; Protein modification; Protein phosphorylation; Bacterial toxins; Biosynthesis
- M.E.Webb@leeds.ac.uk
- Phone
- +44(0)113 343 6423
- Location
- G.16 Chemistry
- Faculty
- Engineering and Physical Sciences
- School
- Chemistry
Introduction
Modification of proteins is central to both their function in the cell and to their exploitation in biotechnology and medicine. Work in the group covers both aspects of protein modification with a particular focus on the formation of covalently-linked cofactors and phosphorylation, and on the development of new methods for site-specific protein modification. We work on a wide variety of protein systems including enzymes from primary and secondary metabolite biosynthesis, protein kinases, bacterial toxins and antibodies. From a biochemical point-of-view, we are determining how post-translational changes to a protein can perturb its function, localisation and structure and how these changes are regulated; from a chemical point-of-view, we are determining the limits to which it is possible to manipulate the structure of native folded proteins with applications in the study of protein dynamics, cellular imaging and biological therapeutics.
Current major projects
- The development of new strategies for protein modification using chemoenzymatic and chemical approaches
- Biosynthesis of primary and secondary metabolites
- Understanding the origin and function of protein post-translational modifications including autocatalytic rearrangement and non-canonical phosphorylation.
Detailed research programme
Development of new strategies for protein modification using chemoenzymatic approaches
The majority of protein labelling approaches used in academia and industry uses a limited subset of chemoselective but non residue-specific labelling chemistries. Our approaches focus on the use of enzymes for the selective and quantitative modification of proteins with a particular focus on application of transpeptidases to enable methods which are near quantitative in the labelling reagents as well as the protein to be labelled. Using this approach we have modified a wide-range of proteins with applications in cellular imaging, drug delivery and protein interaction analysis.
Biosynthesis of primary and secondary metabolites
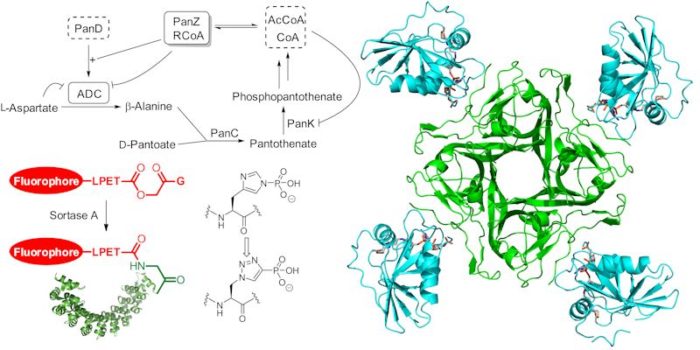
We have long-standing interests in the biosynthesis of vitamin B5 (pantothenate), particularly the enzyme aspartate decarboxylase, and other metabolites. We have used a variety of biophysical (SPR, ITC, SAXS) and structural approaches to characterise the behaviour of these enzymes. More recently, working with Dr Ryan Seipke, we have developed interests in the biosynthesis of peptide and depsipeptide natural products such as the surugamides and antimycins.
Role of post-translational modifications in proteins
Many proteins contain post-translational modifications – these include not only well-known modifications such as phosphorylation but also catalysed changes to the backbone of the peptide to form covalently cofactors. We have particular interests in non-canonical phosphorylation (particularly of phosphohistidine) and have previously developed synthetic approaches including stable analogues of the acid-labile modification to enable antibody production and biophysical characterisation of phosphohistidine-mediated protein-protein interactions.