Dr Peter Adams
- Position
- Associate Professor
- Areas of expertise
- biophysics; photosynthesis; light-harvesting proteins; lipid membranes; fluorescence spectroscopy and microscopy; atomic force microscopy.
- Phone
- +44(0)113 343 9718
- Location
- 8.50aa E C Stoner
- Faculty
- Engineering and Physical Sciences
- School
- Physics
Introduction
My research investigates membrane protein and lipid assembly, with a focus on the specialized “light-harvesting” membranes which are involved in photosynthesis. We wish to understand, mimic and control the organization of membranes, inspired by the “light-harvesting” membranes found within plants (chloroplasts) and photosynthetic bacteria. My group takes a multi-disciplinary approach combining aspects of surface chemistry, nano/micro fabrication, protein biochemistry, spectroscopy and various microscopies.
Current major projects
- What is the role of light-harvesting proteins in the structure and dynamic reorganization of photosynthetic membranes?
- What is the structural basis of photoprotection in plant light-harvesting proteins? (i.e., experiment and theory of energy dissipation in LHCII)
- Development of new controllable model membranes and new quantitative analyses to interrogate light harvesting proteins.
- Development of novel energy-transferring nanomaterials which enhance the photophysical properties of light-harvesting proteins.
Detailed research programme
Biophysics of natural “light-harvesting” membranes of photosynthesis:
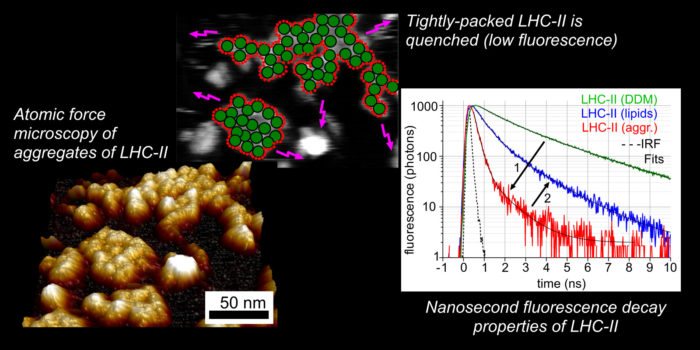
A major goal is to understand the role of plant Light-Harvesting Complex II (LHCII) the process of photoprotection. Photosynthetic “light-harvesting” membranes contain organized domains of membrane proteins which work together as a coordinated network for energy trapping. The plant protein LHCII acts as the major antenna protein for plants, and is possibly the most abundant membrane protein in the world. It has a critical role in safely dissipating energy when light intensity is too high (e.g., on a cold, sunny day). We are studying the molecular mechanism of this photoprotective process (called “nonphotochemical quenching”) by performing experiments and collaborating with theoreticians. We also have a longstanding interest in the LH membranes from purple bacteria and “chlorosome” antennae of green bacteria.
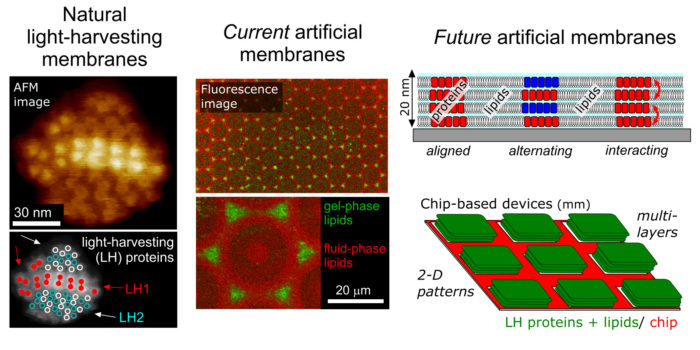
Model biomembranes, artificial photosynthesis and nanotechnology:
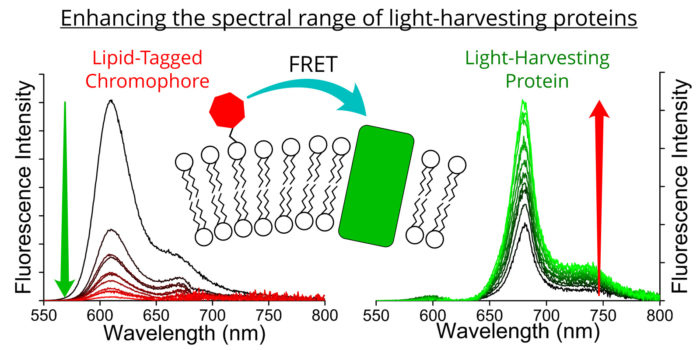
Another goal is the development of novel nanocomposites for energy transfer. Purified LH proteins, natural lipids, synthetic lipids, quantum dots, polymers and other materials are used as building blocks to generate model protein/lipid systems. Using lipid bilayers as a platform, we have shown that we can interface Light Harvesting proteins with a range of different nanomaterials, to study their function and to change (possibly “enhance”) the natural proteins. Relatedly, we are interested in model lipid membranes as a platform to study lipid biophysics and protein function. By mimicking the natural stacked membrane but attaching them to glass or mica surfaces, these model membranes are biologically relevant and also amenable to characterization by advanced microscopy.
Advanced microscopy and spectroscopy of various biophysical samples:
We are interested in using Atomic Force Microscopy (AFM) to understand natural and synthetic membranes and protein arrangements in collaborations with others. AFM has excellent signal-to-noise and can image soft biological samples to sub-nanometre spatial resolution. For example we can observe the arrangement of hundreds of membrane proteins without the need for averaging.
We are also interested in time-resolved fluorescence spectroscopy and fluorescence microscopy (TCSPC and FLIM) to explore the photophysical properties of any interesting biological or nanophotonic materials.