Dr Simon Connell
- Position
- Associate Professor
- Areas of expertise
- AFM; biological physics; nanomechanics; lipid membranes; fibrin
- Phone
- +44(0) 113 343 8241
- Location
- E.C.Stoner Building, 8.32a
- Faculty
- Engineering and Physical Sciences
- School
- Physics
Introduction
My research primarily concerns the development and application of Atomic Force Microscopy (AFM) and related techniques to areas of biological physics and soft matter physics, and to understand fundamental properties of matter by characterising structure, mechanics and dynamics at the nanoscale. Recent developments in high speed AFM have opened a new window into the nanoscale dynamics of biological systems and soft polymers, and we extract quantitative properties from image analysis of the resultant movies with nanometre resolution. A full picture of the system being studied often requires complementary characterisation techniques, and ideally these are carried out simultaneously, such as correlative fluorescence microscopy. Other sequential methods include Quartz-Crystal Microbalance with Dissipation (QCM-D) to quantify surface deposition; magnetic tweezers with high speed cameras for passive and active micro-rheology of soft gels and solutions, Langmuir trough for characterisation of interfacial films and surface deposition, and differential scanning calorimetry (DSC) for the thermal response. I also manage the Leeds EPSRC AFM Facility.
Current major projects
- Lipid bilayers: phase, asymmetry, dynamics
Detailed research programme
Lipid bilayers
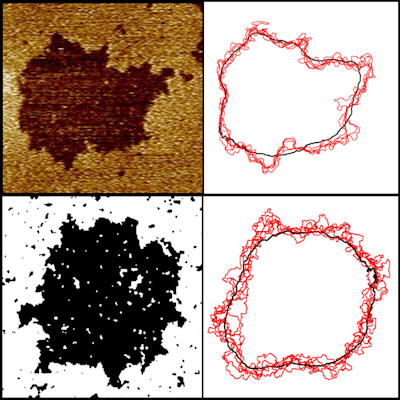
The outer plasma membrane of any cell, and all interior membranes which compartmentalise the cell, consist of lipids, amphiphilic molecules which spontaneously self-assemble into a bilayer. This fluid lamellar phase constitutes an impermeable chemical barrier, and is home to a large proportion of cell proteins, and an area of great interest is how the properties of the membrane affect the functioning of trans-membrane proteins, and vice-versa. For many years the membrane was considered as a passive flexible membrane, but in fact they have their own incredibly rich behaviour, in terms of phase structure, curvature and asymmetry. We are particularly interested in nanodomain structure, formation mechanism and dynamics, related to the idea ‘lipid rafts’.
Fibrinogen and blood clotting
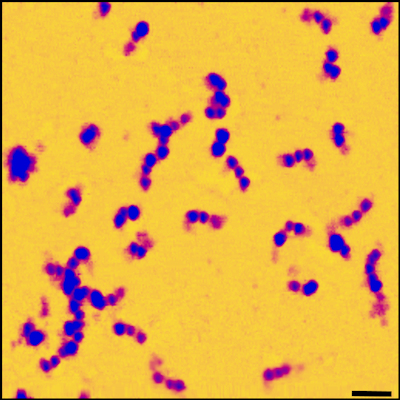
Fibrinogen is a soluble protein monomer in the blood, which upon injury is converted by thrombin-mediated proteolysis into fibrin, a form which polymerises and grows by lateral assembly into long fibres around 100 nm in diameter This can then crosslink via Factor XIII into a strong and flexible network, the basis of the blood clot. Fibrin is a visco-elastic material with remarkable biomechanical properties, including an incredible extensibility of over 300%. We study this mechanical behaviour across all-length scales, from the single molecule, up through single-fibres with AFM pulling and stretching experiments, to the micro-scale with magnetic tweezers equipped with high speed cameras. Molecular structure and clot architecture are determined using AFM and EM, and clot growth and internal fibre architecture are derived from optical light scattering, spectroscopy and confocal scanning fluorescence microscopy.
Biological and soft matter nano-mechanics
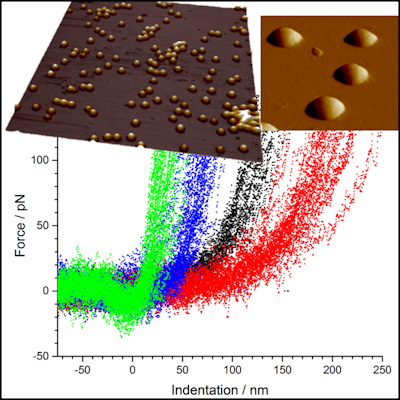
We are developing methods for quantifying mechanical properties of soft materials in the range 1 kPa – 10 GPa, with nanometre resolution using various AFM force mapping techniques. Combining the measurements with temperature control allows spatial discrimination of thermal transitions such as melting point or glass transitions of individual inclusions or phases within complex phase-separated materials. Current projects include determination of the internal mechanical properties of hydrogels and individual nanometre sized microgel particles (both synthetic and protein based) vs temperature and crosslink density; the study of complex food polymer phase structure with correlated micro-RAMAN mapping; and structure and mechanical properties of plant cell walls, particularly the influence of components such as cellulose, callose and xylo-glucans. We also study nano-tribology in soft systems.
Pharmaceutical crystal surface energy and mechanics
We are working on several projects with industrial partners such a Pfizer, AZ, 3M Drug Delivery, Neutek and Interpharm. One major project involves developing a computational approach to predicting the adhesive-cohesive balance between active ingredient and excipients in drug formulation, and then validating this with direct force measurements between opposing crystals. Both simulation and experiment are between known facets of the crystals. Another project involves directly measuring the inter-particulate forces of the various micro-particles which are found in inhalable drugs, informing computational approaches, and linking with advanced 3D imaging of the particulate structure, to help understand de-aggregation and fluidization processes. We are also measuring the strength and fracture mechanics of needle like drug crystals to understand behaviour in packed filtration beds.