Dr Yuan Guo
- Position
- Associate Professor
- Areas of expertise
- glycobiology; multivalency; receptor recognition; signalling; immune regulation
- y.guo@leeds.ac.uk
- Phone
- +44(0)113 343 1420
- Location
- 2.31, Chemistry
- Faculty
- Environment
- School
- Food Science and Nutriton
- Website
- ORCHID
Introduction
Receptors embedded in cell membranes are responsible for cell communication with the outside environment. These receptors interact specifically with their extracellular target ligands and translate such binding signals across the membrane to modulate cellular functions. For many receptors, such extracellular binding events do not induce conformational changes, instead multiple receptors are engaged in binding to multiple ligands found on cell or pathogen surfaces, resulting in receptor cluster formation as a means to generate signals. However, this signalling process is often exploited by pathogens and cancer cells to manipulate immune cell functions to evade immune surveillance.
A key outstanding question is how such external specific ligand binding information is translated into a specific intracellular signal. Our research aims to dissect such specific ligand recognition and signal translation mechanisms so as to provide new insights into the design of specific immune activation or inhibitory ligands for therapeutic purposes. Specifically, we are focusing on a group of calcium dependent glycan binding receptors (also known as C-type lectins) found on immune cell surfaces.
Current major projects
- Reveal general rules governing multivalent lectin-glycan binding affinity and specificity enhancement
- Elucidate how multivalent glycan-lectin binding manipulates dendritic cell (DC) immune function.
- Exploit glycan-nanoparticles for potential therapeutic treatments of allergy and autoimmune diseases.
Detailed research programme
Mechanism of specific, high affinity multivalent lectin-glycan interactions
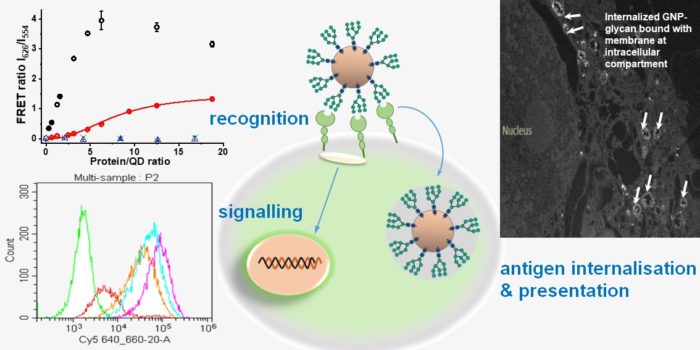
Immune cell surface lectins often form oligomers to cluster their carbohydrate-binding-domains (CRDs) to enhance binding affinity and specificity toward targeting multivalent glycans. This allows them to discriminate between self- and foreign- structures and to trigger pathogen specific immune response.
To understand the specific high affinity recognition, we use two tetrameric lectins: a DC surface receptor DC-SIGN and its closely-related endothelia cell receptor DC-SIGNR as model receptors. We have developed new glycan-nanoparticle based approaches and differentiated their different CRD arrangement and multivalent viral glycan selectivity. Combining carbohydrate synthesis, molecular biology, protein biochemistry and biophysical studies, we are further revealing the thermodynamic and kinetic insights into their multivalent binding affinity enhancement. These understandings will guide potent, high specific lectin targeting glycan-nanoparticles development.
Mechanisms of multivalent glycan-lectin binding regulating DC immune function
DC-SIGN and other lectins (e.g. mannose receptor) are exploited by some virus, bacteria and cancer cells via their surface specific glycan coatings to evade immune surveillance, but the underlying mechanisms remain unclear. To address this problem, we are developing polyvalent glycan nanoparticles of similar size and glycan coatings as virus particles to mimic virus-DC interactions. We are exploiting the nanoparticles’ unique fluorescence and high density properties to reveal binding induced DC surface lectin clustering details, identify the intracellular proteins responsible for signalling and track internalized nanoparticle intracellular routing. Together with the measurement of binding stimulated DC cytokine production, surface co-stimulatory molecules expression and antigen presentation, we aim to reveal the whole picture how glycan-lection stimulation controls DC immune function.
Glycan-nanoparticles for potential therapeutic applications
Together with our clinical collaborators, we are investigating how our glycan-nanoparticles showing strong inhibitory effect on DC immune activation can be harnessed for potential therapeutic treatment against allergy and chronic auto-immune diseases. We are studying how glycan-nanoparticle treatments inhibit DC inflammatory cytokine production, affect allergen presentation for stimulation of T cell responses using clinical samples, paving way to the development of novel glycan-based therapeutic interventions against such chronic immune diseases incurable by current medicines.
