Professor Neil Ranson (Director)
- Position
- Professor of Structural Molecular Biology
- Phone
- +44(0)113 343 7065
- Location
- 10.132 Garstang
- Research Area
- structural biology; cryo-electron microscopy; structural Virology; virus assembly, receptors & uncoating; molecular chaperones; protein misfolding; amyloid; membrane proteins; membrane biogenesis

Introduction
My lab is interested in the structures of large macromolecular assemblies, and particularly in how conformational change in such structures drives biological function. We primarily use cryo-electron microscopy (cryoEM), cryo-electron tomography (cryoET) to determine the structures of such complexes in 3-D. Our current research interests are in three broad areas: structural virology, antimicrobial resistance (especially focused on the outer membrane of Gram-negative bacteria), protein folding and misfolding in vitro and in vivo.
Current major projects
- All aspects of structural virology, including novel virus structure, assembly, receptor binding, and cell entry
- The structure, function and biogenesis of proteins from the outer membrane of Gram-negative bacteria.
- Mechanisms of antibiotic resistance
- The structure of amyloid aggregates and the molecular basis of their cytotoxicity
Detailed Programme
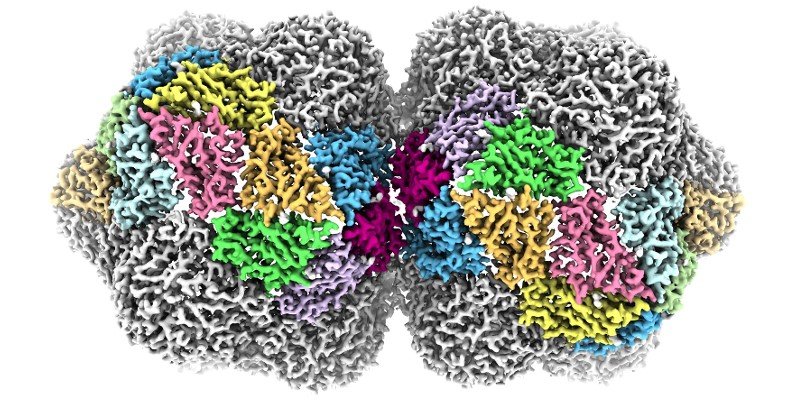
Structural virology
My lab is broadly interested in all aspects of the structure and function virus particles. We have a long-standing interest in discovering the structures of novel plant viruses, and in understanding how these complex macromolecular machines self assemble. I’m also fascinated by the metastability of virus capsids, a crucial evolved characteristic that allows them to be tough enough to survive the extracellular world, but fragile enough to uncoat and allow new cycles of infection once they’ve entered a target cell. We work across multiple types of virus to tackle these interesting aspects of virus biology, including viruses from bacteria, plants, animals and human pathogens.
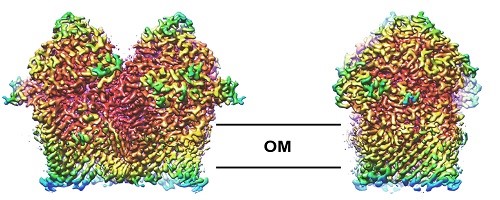
Mechanisms of antimicrobial resistance
A rapidly growing area of interest in my lab is in the structure, function and biogenesis of outer membrane proteins (OMPs) in the outer membrane of Gram-negative bacteria. We are currently working on the structures of a range of outer membrane nutrient transporters that are essential for cell viability and virulence in pathogenic bacteria. We’re also working on the b-barrel assembly machinery (or BAM complex), the OMP complex that is essential for viability, and catalyses the folding pf other OMPs into the lipid bilayer. We’re also looking at mechanisms of antimicrobial resistance in other settings, particularly in how a variety of proteins and natural products impede ribosome function.
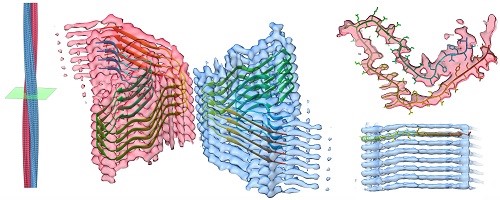
Amyloid biology
The interest in OMP folding described builds upon a career-long fascination with the process of cellular protein folding and misfolding. The final area under active study in my lab further builds on this interest by looking at the structural and cellular consequences of protein misfolding: namely the biology of protein aggregation and amyloid formation. We’re now working on a range of amyloid fibril aggregates and the effects such fibrils have on cells.
Work in my lab is currently funded by BBSRC, Kidney Research UK, MRC, Wellcome and the University of Leeds, and in the past other projects have involved EPSRC and ERC funding to collaborators.
