Professor Alison Baker
- Position
- Professor of Cell and Molecular Biology
- Areas of expertise
- Peroxisomes, Membranes, Transport, Phosphate, Remediation
- A.Baker@leeds.ac.uk
- Location
- Manton 9.05
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
Introduction
Compartmentalisation is a fundamental feature of eukaryotic cells which enables more complex regulation of biochemical processes. However it requires transport systems and processes for exchange of metabolites and proteins between compartments. Peroxisomes are single membrane bound organelles that acquire their constituent proteins via import from the cytosol or in the case of some membrane proteins via an ER derived pathway. Peroxisomes are metabolically versatile and their functions reflect the organism, tissue and even environmental conditions, therefore their function is determined by the proteins they import. Additionally peroxisomes co-operate in metabolism with other organelles so there is an extensive exchange of metabolites. Major efforts within the laboratory are elucidation of protein import mechanisms and characterisation of a peroxisomal ABC transporter which imports substrates for beta oxidation. This is a common peroxisomal metabolic pathway used for mobilisation of storage oil and synthesis of many bioactive molecules in plants. Other interests related to membrane transport processes are in phosphate signalling and transport, both in the context of improving phosphate use efficiency, especially in orphan crops and in use of photosynthetic organisms in remediation of nutrient rich waste water.
Current major projects
- Use of photosynthetic organisms for remediation of nutrient rich waste water
- Structural and biochemical studies of a plant peroxisomal ABC transporter
- Protein import into peroxisomes
Detailed research programme
Use of photosynthetic organisms for remediation of nutrient rich waste water.
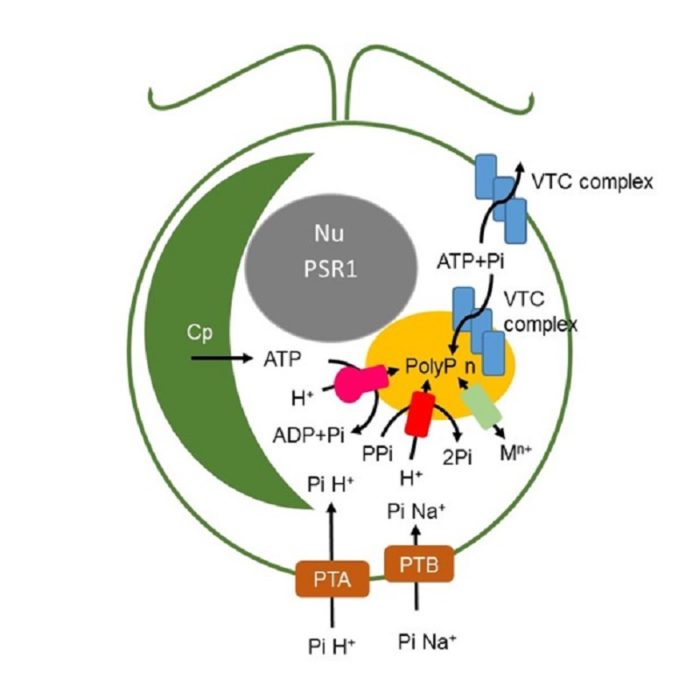
Phosphorus is a limiting nutrient for agriculture in over 50% of cultivated soils. Use of phosphate rich rocks as a source of fertiliser is environmentally and ecologically unsustainable. Human activities result in production of nutrient rich waste waters which if not treated cause eutrophication of water bodies. Current treatments are expensive and not well suited to recovering nutrients for use in agriculture. In collaboration with the School of Civil Engineering in Leeds we are exploring the use of aquatic photosynthetic organisms; duckweed and the model microalga Chlamydomonas reinhardtii in waste water treatment. We have shown that P uptake and growth can be uncoupled in duck weed under temperate climate conditions and we are investigating metabolic manipulations that can enhance P uptake and accumulation in microalgae.
 Structural and biochemical studies of a plant peroxisomal ABC transporter.
Structural and biochemical studies of a plant peroxisomal ABC transporter.
ABC transporters couple ATP hydrolysis to movement of a diverse range of substrates across membranes. Peroxisomes contain ABC transporters which import substrates for beta oxidation. Humans have four peroxisomal ABC transporters which form homodimers with overlapping substrate specficity for fatty acids with different degrees of chain length and unsaturation. S. cerevisae contains one heterodimeric protein whilst plants have just one fused heterodimer which has broad substrate specificity for fatty acids and a wide range of substrates that enter beta oxidation for biosynthesis. We previously showed this protein accepts acyl CoA substrates and cleaves them prior to re-esterification to CoA for metabolism in the peroxisome. Current work is focussed on structure determination of this fascinating protein.
Protein import into peroxisomes.
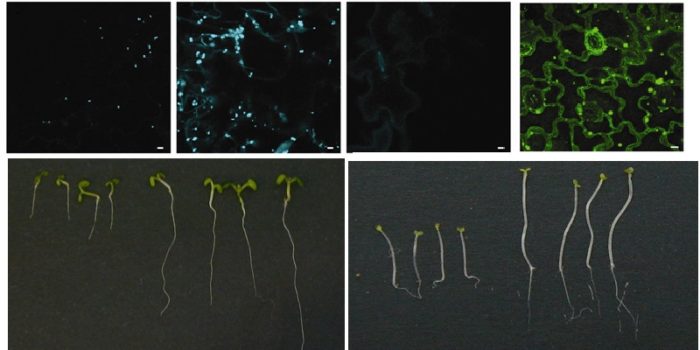
Peroxisomes import proteins via short amino acid targeting motifs known as PTS1 and PTS2. Some proteins have neither a PTS1 nor PTS2 or have a so called non canonical PTS which does not fit the exact consensus. An example of this is catalase and there is increasing evidence that its import and/or subcellular localisation is regulated by environmental conditions. We have been reinvestigating the role of the C terminal region of catalase in protein import and function. In collaboration with the School of Chemistry at Leeds we have been testing chemical probes to interrogate the PTS1 import pathway and have re-engineered the PTS1 protein import pathway to develop designer peroxisomes for synthetic biology.