Professor Bruce Turnbull
- Position
- Professor of Biomolecular Chemistry
- Areas of expertise
- Chemical Biology; Synthetic Glycobiology; Protein Modification
- Phone
- +44(0)113 343 7438
- Location
- G.14 Chemistry
- Faculty
- Engineering and Physical Sciences
- School
- Chemistry
Introduction
The surface of every living cell is covered in a dense forest of carbohydrates known as the glycocalyx. The glycoproteins, proteoglycans and glycolipids that make up the glycocalyx, mediate many important biological interactions that are essential for normal health including fertilisation. However, many viruses and bacteria exploit our cell surface carbohydrates (glycans) as a mechanism to adhere to and enter our cells. Some bacteria also produce protein toxins that also exploit cell surface glycans for cell entry. Changes in cell surface glycosylation has also been recognised as cancer markers and may have potential for targeting tumours. My research spans synthetic chemistry, protein engineering and biophysical studies of protein-glycan interactions. Our aim is to understand the glycobiology of host-pathogen interactions and then to take a synthetic biology approach to re-engineer the protein or glycan components to create targeted delivery systems and diagnostic methods.
Current major projects
- Enzymatic synthesis of modified glycans
- Site-specific chemical and enzymatic synthesis of proteins
- Understanding multivalent protein-carbohydrate interactions
- Synthetic glycobiology – re-engineering bacterial toxins for targeted delivery
Detailed research programme
Enzymatic synthesis of modified glycans
Chemical synthesis of carbohydrates is a laborious and challenging area of synthetic chemistry. The growing availability of glycosyl transferases, and the ancillary enzymes to make nucleotide sugar building blocks, is transforming our ability to make larger complex glycans. My group is part of a UK consortium focussed on expanding these methods to the synthesis of unnatural, modified sugars which will have applications in screening libraries for development of glycomimetic drugs, and in diagnostic arrays for detecting pathogens.
Site-specific chemical and enzymatic synthesis of proteins
Modifying proteins with non-peptidic groups (e.g., fluorophores or PEG) can provide useful tools for biochemical/biophysical experiments and also alter protein properties for therapeutic purposes, e.g. antibody-drug conjugates and conjugate vaccines. Traditional methods for protein derivatisation are not always selective and frequently create mixtures of products. We have implemented several methods for the site-specific modification of proteins, and we are developing improved methods for both chemical and enzymatic ligations, e.g., sortase-mediated ligation.
Understanding multivalent protein-carbohydrate interactions
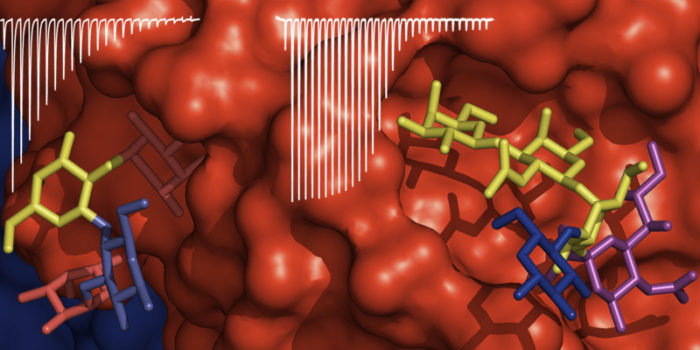
Protein-carbohydrate interactions typically have shallow binding sites and may exhibit very weak affinities with monosaccharides or small glycans. Functional affinities and enhanced binding selectivity arises from multivalent interactions in which multiple glycans (or multiple epitopes on a complex glycan) interact with multiple binding sites on the target protein. We synthesise multivalent glycans based on polymer and protein scaffolds and use them to understand the basis of binding selectivity and to develop inhibitors of protein-glycan interactions. For example, we are interested in how two different sugar binding sites on the cholera toxin each contribute to cell surface binding and their roles in cell entry and how blood groups make affect the severity of disease for cholera patients.
Synthetic glycobiology – re-engineering bacterial toxins for targeted delivery
AB5 bacterial toxins are produced by a range of bacteria that cause diarrheal disease (e.g. cholera toxin from Vibrio cholerae and Shiga-like toxin from E. coli O157). The pentameric B-subunits are not themselves toxic, but instead acts as the delivery vehicles for transporting the toxic A-subunit into their target cells. We are developing site-specific chemical, enzymatic and genetic methods to re-engineer the non-toxic B-subunits, and to replace the A-subunit with a therapeutic cargo for use in targeted delivery applications. For example, when injected into a muscle, these proteins are taken up by motor neurones and transported through along the axon to their cell bodies located in the central nervous system. We are also interested in intracellular trafficking for directing molecules to specific organelles. We also investigate how changing the architecture of these proteins leads to changes in their ability to manipulate biological membranes.