Professor Michelle Peckham
- Position
- Professor of Cell Biology
- Areas of expertise
- Myosin; Actin; Cytoskeleton; Muscle; super-resolution imaging
- Phone
- +44(0)113 343 4348
- Location
- 8.106 Astbury
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
Introduction
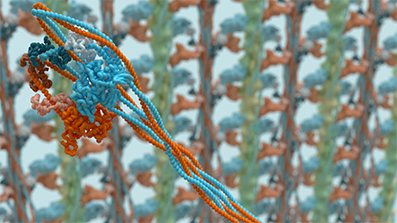
The molecular motor, myosin, is a key protein responsible for muscle contraction and movement in almost every eukaryotic cell. Our current research focuses on these and related cytoskeletal. We research into how their activity is regulated, how they carry out their cellular functions, and effects of disease mutations. We use a wide range of tools, from characterisation of expressed and purified proteins to cultured muscle cells. We are also developing super-resolution imaging (iSIM, STED, PALM and STORM) as tools to investigate proteins are organised within muscle cells. We use Affimer technology for super-resolution imaging, and for detecting specific protein conformations and post-translational modifications for actin and microtubules. We use negative stain and Cryo-EM to investigate myosin structure. The picture shows the structure of shutdown smooth muscle myosin (recently published by our group in Nature, Dec. 2020) obtained by CryoEM.
Current major projects
- How disease mutations affect protein structure and function in skeletal and cardiac myosin,
- How disease mutations affect protein structure and function in non-muscle myosin 2A
- The use of super-resolution imaging techniques (PALM, STED, dSTORM, iSIM) to image the cytoskeleton
- Using Cryo-EM to investigate the structure and regulation of myosins.
Detailed research programme
The cytoskeleton (actin filaments and microtubules) is precisely organised in cells. In skeletal and cardiac muscle, actin and myosin are organised into filaments with precise lengths to power contraction. In non-muscle cells, the actin cytoskeleton is much less well organized, is more dynamic, and these cells contain many different kinds of myosins. The contrast between the organisation and regulation of the cytoskeleton between highly ordered striated muscle, and crawling cells is fascinating. Mutations, or changes to expression levels are important drivers of a wide range of diseases from heart disease to bleeding disorders, and cancer.
Our laboratory uses a wide range of techniques to study muscle differentiation, cancer cell behaviour, the cytoskeleton and myosin including bio-imaging, live cell imaging, protein biochemistry, electron microscopy, crystallography, and a wide range of cell and molecular biological techniques. Collaborations within and beyond the Faculty and the University have involved electron microscopy, NMR, crystallography, optical tweezers, Total Internal Reflection Fluorescence (TIRF) microscopy and most recently super-resolution microscopy. We have built our own 3D PALM/STORM microscope and can image the organisation of proteins in a wide range of cellular structures using this approach to ~5nm precision.
Structure, function, regulation and mutations in Myosin 2
Our lab is interested in the structure and function of myosin 2. As part of this, we investigate the effects of mutations in beta-cardiac myosin that cause heart disease, skeletal muscle myosin mutations that cause myopathies and mutations in non-muscle myosin 2a, which cause bleeding disorders. We are specifically interested in how these mutations affect the structure, function and regulation of these myosins.
To address these questions, we use a combination of techniques. These include circular dichroism to determine effects on secondary structure, and in particular the effects of mutations in the coiled coil tail. We assess the cellular effects of mutations using GFP-tagged myosin expressed in cultured muscle cells, to find out how well the mutant myosin incorporates into muscle sarcomeres. We express and purify full length myosin and myosin domains and use negative stain and Cryo-EM to determine the effects of mutations on structure.
PALM and dSTORM to understand protein organisation in complex structures
We use PALM and dSTORM to investigate the detailed organisation of proteins in structures that are difficult to evaluate by conventional light or electron microscopy. Specifically, we are using this approach to investigate the Z-disc, a narrow (~100nm wide) structure found at the ends of muscle sarcomeres. Its narrow size means that conventional light microscopy is unable to reveal the detailed organisation of proteins within the Z-disc as this is below the resolution limit (~250nm). And yet it contains ~50 different important signalling and structural proteins. How are these proteins organised with respect to each other? To answer this question, we use PALM and dSTORM to determine their organisation, and we have developed novel software to analyse this data (PERPL) to find patterns in our data. In addition, we use Affimers to specifically label proteins in dSTORM, as their small size (~2nm, 10 kDa) means better penetration, a lower linkage error and thus better resolution in our final images.
Other projects in the lab
As part of our research, we are also interested in how the cytoskeleton becomes organised when undifferentiated muscle cells differentiate into multinucleated myotubes. A PhD student in the lab is currently investigating how this process is affected by ‘hard’ and ‘soft’ surfaces, and she is making comparisons with muscle regeneration in vivo. A second PhD student (about to graduate) has been working on the protein ASPM. Mutations in this gene cause microcephaly. It is intriguing to us, because it contains a central region with 81 IQ repeats, and IQ repeats are also found in myosins. These repeats bind light chains, and we are trying to understand how they contribute to the function of ASPM.