Professor Yoselin Benitez-Alfonso
- Position
- Professor of Interdisciplinary Plant Sciences
- Areas of expertise
- signalling; cell-to-cell communication; plasmodesmata; plant development; cell wall biopolymers
- Location
- Miall 9.24
- Faculty
- Biological Sciences
- School
- Biology
Introduction
All multicellular organisms rely on intercellular communication to coordinate growth and responses to physiological and environmental cues. Intercellular communication in plants is physically restricted by the cell wall. Our research focuses on investigating the properties and regulation of cell wall-membranous channels (named plasmodesmata) that connect plant cells and facilitate the symplastic (cytoplasm-to-cytoplasm) transport of proteins, RNAs, viruses, hormones and other signalling molecules. Investigating the molecular composition and physical properties of plasmodesmata is crucial to understand the signalling pathways underpinning plant development and adaptation to the environment. Knowledge on these intriguing structures can be applied in plant biotechnology but can also be used in the development of new sustainable materials, in food science and in healthcare.
Current major projects
- Regulation of symplastic intercellular communication in plant development
- Learning from cell wall micro-domains for the design of novel biotechnological approaches for crop improvement and of new biomaterials
- Exploring beta-1,3 glucans properties as cellulose modifiers, activators of the immune response and in diagnostics of fungal infections.
- Plasmodesmata signalling pathways in the context of legume symbiosis and nitrogen fixation
Detailed research programme
Characterization of cell walls surrounding plasmodesmata and their function in communication
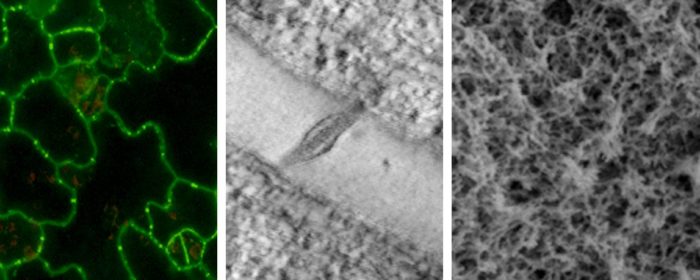
The accumulation of the polymer callose (β-1,3 glucans) in the cell wall constricts the channel aperture, modulating intercellular molecular flux. We are closely studying the proteins involved directly and indirectly in the metabolism of callose at plasmodesmata. We are also dissecting the contribution of other cell wall components in regulating channel structure/aperture. We are also modelling the properties of cell walls around plasmodesmata using composite mixtures and soft polymer physic approaches such as NMR, rheology and AFM nanoindentation. The aim is to link structural and physico-mechanical properties to understand the function of callose and other cell wall biopolymers in regulating the channel transport capacity. This information is essential to manipulate these channels aiming to optimize plant development and environmental responses.
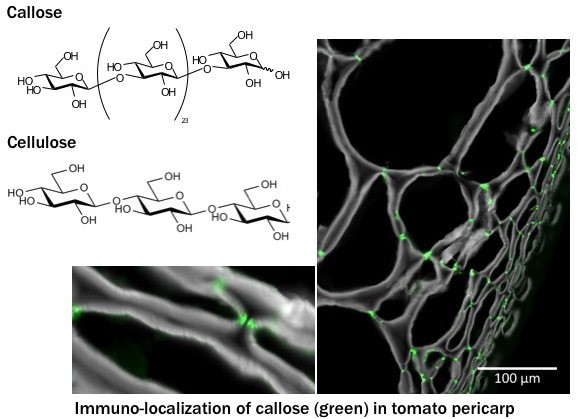
Figure showing structure of callose and cellulose and tomato pericarp tissue labelled with anti-callose (green) and calcofluor white (stain for cellulose). Inset show a magnification of pit field (groups of plasmodesmata).
Dissecting the role of plasmodesmata in the root response to nutrients and abiotic stress conditions
We are using symplastic reporters to study changes in plasmodesmata transport and structure in response to N, P, K and osmotic stress (simulated drought). The aim is to discover factors and components affecting intercellular signalling during the plant response to these stress conditions. Mutants and transgenic lines affecting plasmodesmata permeability will indicate the importance of this pathway in concerting stress responses and highlight new avenues for strategic modifications of root traits to improve plant resilience to climate change.
Manipulating signalling cues associated to legumes-rhizobia symbiosis and nitrogen fixation
We are currently investigating how the expression of plasmodesmata proteins affect the formation of nodules in Medicago truncatula roots and nitrogen-fixing symbiosis. We have observed that when we open plasmodesmata, nodulation is improved (Gaudioso-Pedraza et al., Current Biology 2018). The aim is to understand the role of callose and plasmodesmata in regulating the formation of diverse lateral organs and the plant response to nitrogen depleted and sufficient conditions.
Exploiting knowledge on β-1,3 glucans to design new biomaterials and in diagnostic of fungal infections
Cell wall polymers occur at plasmodesmata in combination with cellulose and play a function in regulating transport capacity. New polymer mixtures are studied for their properties and this knowledge is applied in the manufacture of cellulosic materials. We found how interactions callose/cellulose can modify the properties of polymer mixtures (Abou-Saleh et al., Nature Communications, 2018) and now are using this information to design hydrogels with novel properties. New probes (e.g. monoclonal antibodies) were designed to study β-1,3 glucans in planta but these polymers are highly enriched in fungal cell walls. We are collaborating with the Sheffield and Leeds Teaching Hospitals to exploit these molecular tools in the diagnostic of invasive fungal diseases.