Pore-like proteins designed from scratch
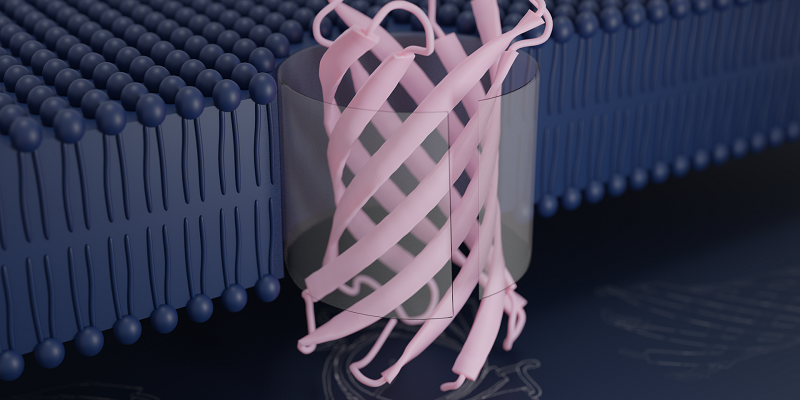
Scientists at Leeds are part of an international collaboration that has designed a protein that self-assembles into an artificial pore.
The protein sequence spontaneously transforms into a "transmembrane beta-barrel" – a tiny conduit or pore that embeds itself into a lipid membrane, mimicking its natural counterparts which are found in the walls of bacterial cells and enable nutrients, vitamins and strands of DNA to pass through.
There is intense scientific interest in the self-assembly of artificial pores as a way of exploiting the way nature moves "cargo" in and out of cells, for use in drug delivery or filtration technologies.
Professor Sheena Radford FRS said: “Proteins are the molecular machines that carry out functions necessary to sustain life. Science has a lot to learn from the way they work, and there has been significant progress in designing bespoke proteins – but up to now, it has not been possible to design a protein that would form a transmembrane beta-barrel. What has been achieved in this study is a major advance.”
Read the full press release on the University of Leeds website
Read "De novo design of transmembrane β barrels" on the Science website
